 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
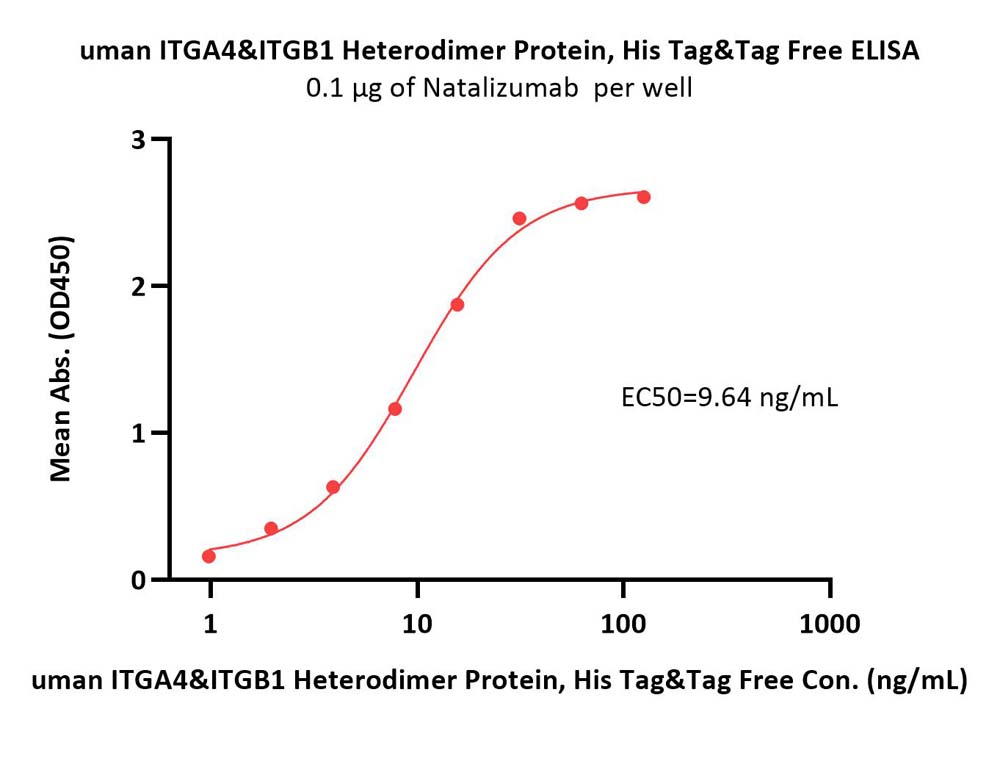
Immobilized Natalizumab at 1 μg/mL (100 μL/well) can bind Human ITGA4&ITGB1 Heterodimer Protein, His Tag&Tag Free (Cat. No. IT1-H52W1) with a linear range of 1-16 ng/mL (QC tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Carotegrast methyl | AJM-300 | Approved | Ea Pharma, Eisai Co Ltd | Carogra | Japan | Colitis, Ulcerative | Ea Pharma Co Ltd | 2022-03-28 | Colitis, Ulcerative | Details |
| Natalizumab | BG-0002; TY-21.6; AN-10022; BG-00002; AN-100226; BG-0002-E | Approved | Biogen Inc, Perrigo Llc | Tysabri, Antegran, Antegren | Japan | Multiple Sclerosis | Biogen Inc | 2004-11-23 | Multiple Sclerosis, Relapsing-Remitting; Epilepsies, Partial; Arthritis, Rheumatoid; Graft vs Host Disease; Stroke; Multiple Sclerosis, Chronic Progressive; Multiple Sclerosis; Multiple Myeloma; Demyelinating Diseases; Myositis, Inclusion Body; Crohn Disease | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| RBx-7796 | RBx-7796 | Clinical | Sun Pharmaceutical Industries Ltd | Rhinitis, Allergic; Asthma | Details |
| ATL-1102 | ATL-1102; TV-1102; ATL/TV-1102; ISIS-107248 | Phase 2 Clinical | Ionis Pharmaceuticals Inc | Multiple Sclerosis; Muscular Dystrophy, Duchenne | Details |
| 7HP-349 | 7HP-349; 7-HP-349 | Details | |||
| Firategrast | T-0047; SB-683699 | Mitsubishi Tanabe Pharma | Details | ||
| Natalizumab biosimilar (Polpharma Biologics) | PB-006 | Phase 3 Clinical | Polpharma Biologics Sa | Multiple Sclerosis, Relapsing-Remitting | Details |
| LLP2A alendronate | Phase 1 Clinical | University Of California | Osteonecrosis; Bone Diseases, Metabolic; Osteoporosis; Bone Diseases | Details |
This web search service is supported by Google Inc.